Vector Borne Diseases
1. MALARIA
Malaria is a protozoal disease caused by infection with parasites of the genus plasmodium and transmitted to man by female anopheles mosquito. It is a very important public health problem in India particularly due to Plasmodium falciparum which is prone to various complications.
Agent: Malaria in man is caused by Plasmodium vivax, Plasmodium falciparum, Plasmodium ovale and Plasmodium malaria. Out of these, Plasmodium vivax and Plasmodium falciparum are very common in India including Maharashtra.
Mode of transmission
Direct – through blood or plasma.
Vectors – bite of female anopheles’ mosquito.
Incubation Period
The duration varies with species of parasite
12 (9-14) days for falciparum malaria.
14 (8-17) days for vivax malaria
When to suspect
The typical attack comprises three distinct stages viz. cold stage, hot stage and sweating stage.
Cold Stage:
The onset is with lassitude, headache, nausea and chilly sensation followed in an hour or so by rigors. The temperature rises rapidly to 39-41°C. Headache is often severe and commonly there is vomiting. In early part of this stage, skin feels cold; later it becomes hot. Parasites are usually demonstrable in the blood. The pulse is rapid and may be weak. This stage lasts for ¼-1 hour.
Hot Stage:
The patient feels burning hot and casts off his clothes. The skin is hot and dry to touch. Headache is intense but nausea commonly diminishes. The pulse is full and respiration rapid. This stage lasts for 2 to 6 hours.
Sweating Stage:
Fever comes down with profuse sweating. The temperature drops rapidly to normal and skin is cool and moist. The pulse rate becomes slower; patient feels relieved and often falls asleep. This stage lasts for 2-4 hours.
The febrile paroxysms occur with definite intermittent periodicity repeating every third or fourth day depending upon the species of the parasite involved. The classical 3 stages (cold, hot and sweating) may not always be
observed due to maturation of generations of parasite at different times. The disease has a tendency to relapse and is characterized by enlargement of the spleen and secondary anemia.
In patients with P. falciparum infection the primary fever in its first few days is usually irregular or even continuous and then the classical 48-hour periodicity becomes established or the fever may continue to be irregular and the hot and cold stages, so typical of other malarial infections are less clearly separated from one another, in persons with poor immunity. The paroxysms are associated with marked prostration. Headache, nausea and vomiting are usually more severe, and there is greater tendency towards the development of delirium, hemolytic jaundice and anemia. The mortality is much greater than in other forms of malaria.
With P. vivax infection, symptoms are same but are usually milder and more regularly divided into “hot” and “cold” stages than in P. falciparum infections.
Complications
The complications of P. falciparum malaria are cerebral malaria, acute renal failure, liver damage, gastro-intestinal symptoms, dehydration, collapse, anemia, black water fever etc. The complications of P. vivax, infection are anemia, splenomegaly, enlargement of liver, herpes, renal complications, ARDS etc.
Investigations
• Diagnosis of Malaria: One of the above clinical features, supported by blood smear examination for malarial parasites.
• Fever with splenomegaly in a patient with the above-mentioned clinical features make diagnosis of malaria more likely.
• Confirmation of diagnosis always depends on seeing the parasite in the blood. In all cases, thick and thin smears should be examined.
• Blood smears may be negative in severe and chronic forms and this would need repeated smears.
Diagnosis of Malaria
• It is stressed that all fever cases should be suspected of malaria after ruling out other common causes and should be investigated for confirmation of malaria by Microscopy or Rapid Diagnostic Kit (RDK) so as to ensure treatment with full therapeutic dose with appropriate drug to all confirmed cases. Presumptive treatment of malaria with a single dose of chloroquine has been stopped.
• All fever cases diagnosed as malaria by either RDT or microscopy should be promptly given effective treatment. The medicine chosen will depend upon whether the patient has vivax malaria or falciparum malaria as diagnosed by the blood test. The flow charts in different settings for diagnosis and drug selection for the treatment of malaria are mentioned below.
Where microscopy result is available within 24 hours
ACT-AL - Artemisinin-based Combination Therapy- Artemether - Lumefantrine
ACT-SP- Artemisinin-based Pyrimethamine) CQ - Chloroquine
PQ - Primaquine
Where microscopy result is not available within 24 hours and Bivalent RDT is used
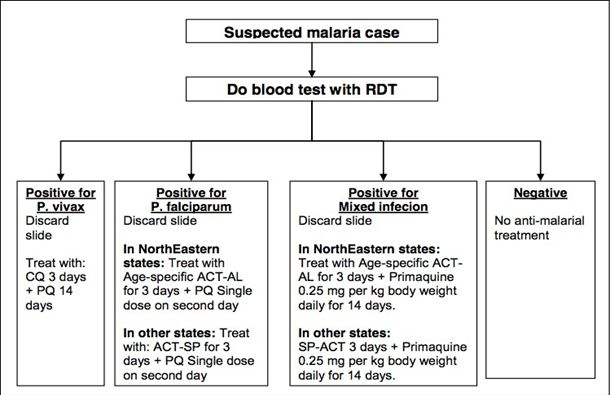
Note: 1) However, if malaria is strongly suspected, prepare & send slide for microscopy
2) If a patient has severe symptoms at any stage, then immediately refer to a nearest PHC or other health facility with indoor patient management or a registered medical doctor.
3) PQ is contra-indicated in pregnancy and in children under 1 year (Infant).
Note: PQ is contra-indicated in pregnancy and in children under 1 year (Infant).
ACT-AL -Artemisinin-based Combination Therapy: Artemether - Lumefantrine ACT-SP-Artemisinin-based Combination Therapy (Artesunate+Sulfadoxine- Pyrimethamine)
CQ - Chloroquine PQ - Primaquine
TREATMENT OF MALARIA
Antimalarial drugs used in public health in India:
· Schizonticidal drugs: Chloroquine, quinine, sulfadoxine-pyrimethamine, artemisinin derivatives (artesunate, arte-mether and arte-ether).
· Gametocytocidal and anti-relapse drug: Primaquine.
All fever cases diagnosed as malaria by RDT or microscopy should promptly be given effective treatment. The treatment will depend upon the species of Plasmodium diagnosed.
A. Treatment of P. falciparum malaria
Artemisinin Combination Therapy (ACT) should be given to all the confirmed P. falciparum cases found positive by microscopy or RDT.
ACT consists of an artemisinin derivative combined with a long- acting antimalarial (amodiaquine, lumefantrine, mefloquine, piperaquine or sulfadoxine- pyrimethamine). The ACT recommended in the National Programme all over India except northeastern states is artesunate (4 mg/kg body weight) daily for 3 days and sulfadoxine (25 mg/kg body weight) -pyrimethamine (1.25 mg/kg body weight) [AS+SP] on Day 0.
In the northeastern states (Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, and Tripura), due to the recent reports of late treatment failures to the current combination of AS+SP in P. falciparum malaria, the presently recommended ACT in national drug policy is fixed dose combination (FDC) of Artemether-lumefantrine (AL).
In Other States (other than North-Eastern States):
1. Artemisinin based Combination Therapy (ACT-SP)* Artesunate 4 mg/kg body weight daily for 3 days Plus
Sulfadoxine (25 mg/kg body weight) – Pyrimethamine (1.25 mg/kg body weight) on first day.
* ACT is not to be given in 1st trimester of pregnancy.
2. Primaquine*: 0.75 mg/kg body weight on day 2.
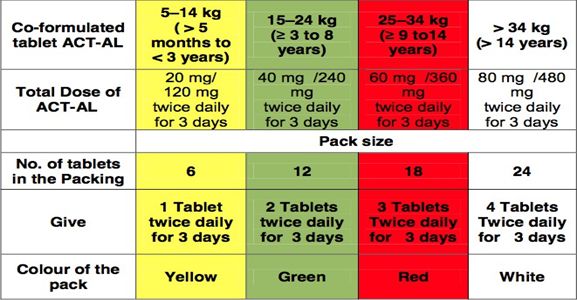
With the introduction of different colored Blister Packs for different age groups, treatment by the field level staff has been made easy. The color code for different age groups for Packing of Tablet ACT+SP has been given as follows:
Dosage Chart for Treatment of falciparum Malaria with ACT-SP
Age group (years) | 1st day | 2nd day | 3rd day | ||
AS | SP | AS | PQ | AS | |
0-1 Pink Blister | 1 (25 mg) | 1 (250mg+12.5m g) | 1 (25 mg) | o | 1 (25 mg) |
1-4 Yellow Blister | 1 (50 mg) | 1 (500mg+25mg) | 1 (50 mg) | 1 (7.5mg base each) | 1 (50 mg) |
5 -8 Green Blister | 1 (100 mg) | 1 (750mg+37.5m g) | 1 (100 mg) | 2 (7.5mg base each) | 1 (100 mg) |
9-14 Red Blister | 1 (150 mg) | 2 (500mg+25mg) | 1 (150 mg) | 4 (7.5mg base each) | 1 (150 mg) |
15 & above White Blister | 1 (200 mg) | 2 (750mg+37.5m g) | 1 (200 mg) | 6 (7.5mg base each) | 1 (200 mg) |
* SP is not to be prescribed for children <5 months of age and should be treated with alternate ACT
In North-Eastern States (NE States):
1. ACT-AL Co-formulated tablet of: Artemether (20 mg) - Lumefantrine (120 mg) (Not recommended during the first trimester of pregnancy and for children weighing < 5 kg)
2. Primaquine*: 0.75 mg/kg body weight on day 2
Recommended regimen by weight and age group
The packing size for different age groups based on Kg bodyweight
Treatment of uncomplicated P. falciparum cases in pregnancy 1st Trimester: Quinine salt 10mg/kg 3 times daily for 7 days.
Quinine may induce hypoglycemia; pregnant women should not start taking quinine on an empty stomach and should eat regularly, while on quinine treatment.
2nd and 3rd Trimesters: Area-specific ACT as per dosage schedule given below. i.e.
ACT-AL in North Eastern States ACT-SP in Other States
*Primaquine should not be given in pregnancy.
B. Treatment of P. vivax malaria
Confirmed P. vivax cases should be treated with chloroquine in full therapeutic dose
Chloroquine: 25 mg/kg body weight divided over three days i.e.
10 mg/kg on day 1,
10 mg/kg on day 2 and
5 mg/kg on day 3.
Dosage Chart for Treatment of Vivax Malaria
Primaquine: 0.25 mg/kg body weight daily for 14 days.
Primaquine is contraindicated in infants, pregnant women and individuals with G6PD deficiency.
14 day regimen of Primaquine should be given under supervision.
Note: CQ 250mg tablet is having 150 mg base
C. Treatment of mixed infections (P. vivax + P. falciparum) cases:
All mixed infections should be treated with full course of ACT and Primaquine 0.25 mg per kg body weight daily for 14 days.
In North-Eastern States: Treat with: Age-specific ACT-AL for 3 days + Primaquine
0.25 mg per kg body weight daily for 14 days.
In Other States: SP-ACT 3 days + Primaquine 0.25 mg per kg body wt. daily for 14 days.
Dosage Chart for Treatment of mixed (vivax and falciparum) Malaria with ACT-SP
Age | Day 1 | Day 2 | Day 3 | Day 4 to 14 | |||
CQ (150 mg base) | PQ (2.5 mg) | CQ (150 mg base) | PQ (2.5 mg) | CQ (150 mg base) | PQ (2.5 mg) | PQ (2.5 mg) | |
Less than 1 year | ½ | 0 | ½ | 0 | ¼ | 0 | 0 |
1 – 4 years | 1 | 1 | 1 | 1 | ½ | 1 | 1 |
5 – 8 years | 2 | 2 | 2 | 2 | 1 | 2 | 2 |
9 – 14 years | 3 | 4 | 3 | 4 | 1½ | 4 | 4 |
15 years or more | 4 | 6 | 4 | 6 | 2 | 6 | 6 |
Pregnancy | 4 | 0 | 4 | 0 | 2 | 0 | 0 |
*All cases of mixed infection are to be treated as Pf as per the drug policy applicable in the area plus primaquine for 14 days.
D. Treatment of severe malaria cases
Severe malaria is an emergency and treatment should be given as per severity and associated complications, which can be best, decided by the treating physicians. Before admitting or referring patients, the attending doctor or health worker, whoever is able to do it, should do RDT and take blood smear; give a parenteral dose of artemisinin derivative or quinine in suspected cerebral malaria cases and send case sheet, details of treatment history and blood slide with patient. Parenteral artemisinin derivatives or quinine should be used irrespective of chloroquine resistance status of the area with one of the following options:
Chemotherapy of severe and complicated malaria
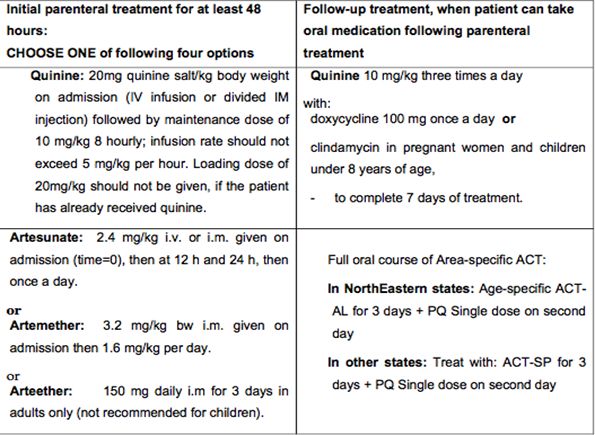
Note: The parenteral treatment in severe malaria cases should be given for minimum of 24 hours once started (irrespective of the patient’s ability to tolerate oral medication earlier than 24 hours).
After parenteral artemisinin therapy, patients will receive a full course of Area-specific oral ACT for 3 days. Those patients who received parenteral Quinine therapy should receive oral Quinine 10 mg/kg body weight three times a day for 7 days (including the days when parenteral Quinine was administered) plus Doxycycline 3 mg/kg body weight once a day or Clindamycin 10 mg/kg body weight 12-hourly for 7 days (Doxycycline is contraindicated in pregnant women and children under 8 years of age) or area-specific ACT as described.
Note:
· Pregnant women with severe malaria in any trimester can be treated with artemisinin derivatives, which, in contrast to quinine, do not risk aggravating hypoglycaemia.
· The parenteral treatment should be given for minimum of 48 hours
· Once the patient can take oral therapy, give:
▶ Quinine 10 mg/kg three times a day with doxycycline 100 mg once a day or clindamycin in pregnant women and children under 8 years of age, to complete 7 days of treatment, in patients started on parenteral quinine.
▶ Full course of ACT to patients started on artemisinin derivatives.
· Use of mefloquine should be avoided in cerebral malaria due to neuropsychiatric complications associated with it.
E. Chemoprophylaxis:
Chemoprophylaxis should be administered only in selective groups in high
P. falciparum endemic areas. Use of personal protection measures including Insecticide Treated bed Nets (ITN) / Long Lasting Insecticidal Nets (LLIN) should be encouraged for pregnant women and other vulnerable population including travelers for longer stay. However, for longer stay of Military and Para-military forces in high Pf endemic areas, the practice of chemoprophylaxis should be followed wherever appropriate e.g., troops on night patrol duty and decisions of their Medical Administrative Authority should be followed.
e.1. Short term chemoprophylaxis (up to 6 weeks) Doxycycline: 100 mg once daily for adults and
1.5 mg/kg once daily for children (contraindicated below 8 years)
The drug should be started 2 days before travel and continued for 4 weeks after leaving the malarious area.
Note: It is not recommended for pregnant women and children less than 8 years.
e.2. Chemoprophylaxis for longer stay (more than 6 weeks)
Mefloquine: 250 mg weekly for adults and should be administered two weeks before, during and four weeks after exposure.
Note: Mefloquine is contraindicated in individuals with history of convulsions, neuropsychiatric problems and cardiac conditions. Therefore, necessary precautions should be taken and all should undergo screening before prescription of the drug.
2. DENGUE
Dengue is the most important emerging tropical viral disease of human beings in the world today. All four dengue virus (Dengue 1, 2, 3 and 4) infections may be asymptomatic, may lead to dengue fever (DF), dengue haemorrhagic fever (DHF) or when associated with plasma leakage may lead to hypovolemic shock and dengue shock syndrome (DSS).
Salient features:
Common Conditions
· Dengue fever is an acute febrile illness of 2-7 days with two or more of the following manifestations. Headache, retro orbital pain, myalgia, arthralgia, rash.
· Haemorrhagic manifestation (petechiae and positive tourniquet test) and Leucopenia
Dengue hemorrhagic fever (DHF), if one or more of the following are present
· Positive tourniquet test
· Petechiae, purpura or ecchymosis
· Bleeding from mucosa
· Haematemesis, melena
· Thrombocytopenia (platelets 100,000 cells/mm3 or less) and evidence of plasma leakage.
Dengue shock syndrome (DSS)
· All the above criteria of DHF plus signs of circulatory failure.
Notes: The tourniquet test is performed by inflating a blood pressure cuff to mid-way between the systolic and diastolic pressure.
Non pharmacological treatment
· Bed rest is advisable during the acute phase.
· Use cold sponging to keep temperature below
Pharmacological treatment
· Management of dengue fever is symptomatic and supportive
· Antipyretics may be used to lower the body temperature. Aspirin/NSAIDs like ibuprofen etc should be avoided since it may cause gastritis, vomiting, acidosis and platelet dysfunction.
· Paracetamol is preferable in the doses as follows:
· 1-2 years: 60 –120 mg/dose
· 3-6 years: 120 mg/dose
· 7-12 years: 240 mg/dose
· Adult : 500mg/dose
Note: In children the dose is calculated as per 10mg/kg body weight per dose which can be repeated at the int
· erval of 6 hrs.
· Oral fluid and electrolyte therapy are recommended for patients with excessive sweating or vomiting.
· Patients should be monitored in DHF endemic area until they become afebrile for one day without the use of antipyretics and after platelet and haematocrit determinations are stable, platelet count is more than 50,000/mm .
Management of Dengue Haemorrhagic Fever (Febrile Phase)
· The management of febrile phase is similar to that of DF.
· Paracetamol is recommended to keep the temperature below 39OC. Copious amount of fluid should be given orally, to the extent the patient tolerates, oral hydration solution (ORS), such as those used for the treatment of diarrhoeal diseases and/or fruit juices are preferable to plain water
· IV fluid may be administered if the patient is vomiting persistently or refusing to feed.
· Patients should be closely monitored for the initial signs of shock. The critical period is during the transition from the febrile to the afebrile stage and usually occurs after the third day of illness.
· Serial haematocrit determinations are essential guide for treatment, since they reflect the degree of plasma leakage and need for intravenous administration of fluids.
· Haematocrit should be determined daily from the third day until the temperature has remained normal for one or two days. If haematocrit determination is not possible, haemoglobin determination may be carried out as an alternative.
· The details of IV treatment when required for patients are given in Figure
Management of DHF Grade I and Grade II:
· Any person who has dengue fever with thrombocytopenia and haemoconcentration and presents with abdominal pain, black tarry stools, epistaxis, bleeding from the gums and infection etc needs to be hospitalized.
· All these patients should be observed for signs of shock. The critical period for development of shock is transition from febrile to afebrile phase of illness, which usually occurs after third day of illness.
· A rise of haemoconcentration indicates need for IV fluid therapy. If despite the treatment, the patient develops fall in BP, decrease in urine output or other features of shock, the management for Grade III/IV DHF/DSS should be instituted.
· Oral rehydration should be given along with antipyretics like paracetamol sponging, etc. as described above.
· The detailed treatment for patient with DHF Grade I and II is given at Figure 5. Common signs of complications are observed during the afebrile phase of DHF. Immediately after hospitalization, the haematocrit, platelet count and vital signs should be examined to assess the patient’s condition and intravenous fluid therapy should be started. The patient requires regular and sustained monitoring.
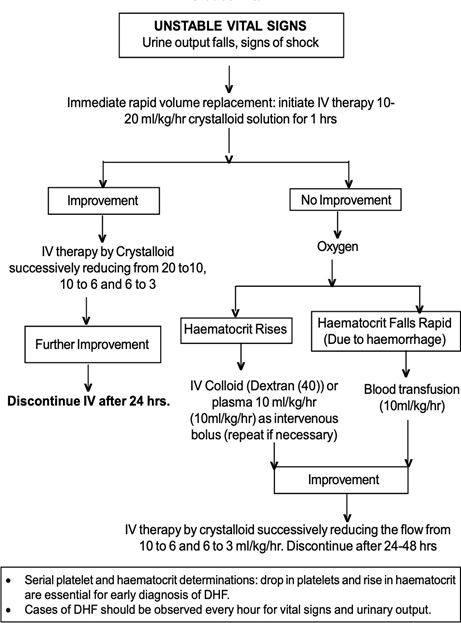
Fluid requirement
· The volume of fluid required to be replaced should be just sufficient to maintain effective circulation during the period of plasma leakage. To ensure adequate fluid replacement and avoid over-fluid infusion, the rate of intravenous fluid should be adjusted throughout the 24 to 48 hour period of plasma leakage by periodic haematocrit determinations and assessment of vital signs.
· The required regimen of fluid should be calculated on the basis of body weight and charted on a 1-3 hourly basis, or more frequently in the case of shock. The flow of fluid and the time of infusion are dependent on the severity of DHF. The schedule given below is a guideline and calculated for moderate dehydration of about 6% deficit (plus maintenance) (Table 5).
Table: Fluid requirement as per body weight of the patient
Weight on admission (kg) | Fluid requirement/kg body weight/day (ml/kg) |
<7 | 220 |
7-11 | 162 |
12-18 | 130 |
>18 | 90 |
In older children who weigh more than 40 kg, the volume needed for 24 hours should be calculated as twice that required for maintenance (using the Holiday and Segar formula). The maintenance fluid should be calculated as follows:
Maintenance fluid requirement according to Holiday and Segar formula
For a child weighing 40 kgs, the maintenance is: 1500 + (20x20) = 1900 ml. This means that the child requires 3800 ml IV fluid during 24 hours.
Indications of red cell transfusion
· Loss of blood (overt blood) -10% or more of total blood volume
· Preferably whole blood/ component to be used
· Refractory shock despite adequate fluid administration and declining haematocrit - replacement volume should be 10 ml/kg body weight at a time and coagulogram should be done.
· If fluid overload is present, PCV is to be given
Indications of platelet transfusion
· Prophylactic platelet transfusion may be given at level of less than 10,000 cells/mm3 in absence of bleeding manifestations.
· Prolonged shock; with coagulopathy and abnormal coagulogram.
· In case of systemic massive bleeding, platelet transfusion may be needed in addition to red cell transfusion.
3. CHIKUNGUNYA
Chikungunya is caused by an alpha virus closely related to O’ nyong – nyong virus. The main vector is Aedes aegypti mosquito.
Salient features
· Acute self-limiting illness Incubation period is of 2 to 4 days. Abrupt onset presenting as fever with severe joint pain
· After 1 – 4 days, fever subsides, there will be a afebrile period 3 days, fever returns with an itching maculopapular rash on trunk and extensor surfaces of limbs.
· After another 3-6 days fever subsides and there is complete recovery.
· Crippling arthropathy can occur intermittently for up to 4 months, in some cases even
· up to 5 years.
Non pharmacological treatment
· Mild exercises and physiotherapy may be suggested in recovering persons.
· Exposure to warm environment may be suggested
· Non weight bearing exercises
· Surgery in severely damaged joints
Pharmacological treatment
· Acute stage of illness
· Treat symptomatically - Tab. paracetamol 1 gm 3-4 times a day for fever, headache and pain
· Avoid aspirin and steroid because of risk of GI side effects and Reye’s
syndrome with aspirin.
· Tab. hydroxychloroquine 200mg orally once daily or Tab. chloroquine phosphate 300mg per day for a period of 4 weeks in cases where arthralgia is refractory to other drugs.
· Side effects of hydroxychloroquine include retinal damage and elevated liver enzymes.
References
No references available